Juniper Publishers-Journal of Ophthalmology
Abstract
Purpose
To evaluate clinical safety and
efficacy of a novel use of an ocular tissue adhesive in Descemet’s
Stripping (Automated) Endothelial Keratoplasty (DSAEK).
Methods
35 consecutive DSAEK cases were
evaluated retrospectively. In group-A (nA=15) the tissue adhesive (Re
Sure Adherent Ocular Bandage, Ocular Therapeutix, Inc., Bedford, MA) had
been used, prior to placement of one suture, while in group-B (nB=20),
only nylon sutures were used for the closure of corneal incisions.
Peri-operative complications were noted. Visual Acuity, refraction and
topographic cylinder, Intraocular Pressure (IOP), and endothelial cell
counts (ECC) were monitored long-term for up to two years.
Results
Follow-up time was 10.5±8.5 (8 to
29) months. No case from group-A required any additional air insertion
following the tissue adhesive application and no case required
additional intra operative surgical manipulation for further graft
centration. In group-B eighteen (out of twenty) cases required
intra-operative supplemental air insertion, and four of those
intra-operative repositioning of the graft. The differences in visual
acuity and IOP were not statistically significant; ECC change of -16% in
group-A vs. -21% was noted in group- B (statistically significant, p
=0.03). Hyperopic shift was noted in both groups; cylinder reduction was
noted, too, with group-A performing better.
Conclusions
Tissue adhesive may be a valuable
adjunct in clear-cornea DSEAK by stabilizing the potential of air
escape from the main incision inadvertently occurring during suture
placement.
Keywords: LED Cassini;
multi-color LED topography; Acanthamoeba keratitis; Point-source
topography; Pentacam HR; Placido topography; Scheimpflug topometry;
Differential topography; Irregular corneal astigmatism; Stray light
measurements; C-Quant; Anterior-Segment Optical Coherence Tomography
Introduction
Descemet’s Stripping (Automated) Endothelial
Keratoplasty (DSAEK) surgery has become the standard of care worldwide
for endothelial dysfunction management [1] superseding for this purpose
Penetrating Keratoplasty (PK). PK involves a full corneal thickness,
open-chamber procedure; among the disadvantages noted with this
procedure are the prolonged visual rehabilitation, unpredictable
cylindrical refractive changes (high postoperative astigmatism),
susceptibility to ocular surface complications (wound dehiscence), and
vulnerability to traumatic wound rupture [2,3].DSAEK, introduced 2002,
[4] involves the posterior cornea lamellae in a closed-chamber procedure
[5]. Because of this, it is considered safer, provides faster visual
recovery, [6] usually requires only few sutures and causes less
astigmatic change,[7] overcoming some of the limitations of PK.
The corneal incisions associated with ocular corneal
surgery, such as cataract and lamellar keratoplasty, are becoming
smaller, depending on surgical instrumentation and techniques, as well
as on implantation materials and designs. There is, nevertheless,
concern that closure of DSAEK incisions with nylon sutures may induce
air-bubble escape and possible graft slippage. Additionally, astigmatic
changes may affect visual function. We have observed during our
experience with DSAEK [8] topographic and tomographic changes consistent
with significant irregular astigmatism along the incision site.
Application of ocular tissue adhesives for the
closure of corneal incisions in DSAEK is considered a novel approach.
This technique carries the benefits of an absence of risk of
intraoperative needle stick injury and later suture removal. The purpose
of this study was to evaluate the clinical efficacy of ocular tissue
adhesive application in DSAEK cases.
Materials and Methods
This retrospective case series study received
approval by the Ethics Committee of our Institution, adherent to the
tenets of the Declaration of Helsinki. Informed written consent for the
anonymous use of data had been obtained from each subject at the time of
the first clinical visit or prior to the operation.
Inclusion Criteria
All consecutive DSEAK cases in our institution were
considered for this study. The decision to proceed with ocular tissue
adhesive-assisted closure (group-A, nA= 15 eyes) or traditional
nylon-suture closure (group-B, nB= 20 eyes) or was based on random
choice (coin toss). No case included in the study involved concurrent
cataract removal and/or intraocular lens replacement surgery, which in
our clinical practice corresponds to near 1/3 of the DSAEK operations.
All cases were performed by the same surgeon (AJK), as an ambulatory
outpatient surgery procedure (not requiring hospital admission), and
under monitored local anesthesia with peribulbar block [9].
Surgical Technique
We prepared the DSEAK grafts ourselves, with a Moria
artificial chamber (Moria Surgical, Antony, France), an LSK (Gebauer
Medizintechnik GmbH, Enzkreis, Germany) microkeratome (350 μm
microkeratome head), and a Hanna suction punch block (Moria). Typical
central graft thickness was of the order of 120 μm. A main clear cornea
4.50-mm incision at the 9th hour was employed for implantation using the
singleuse Busin glide spatula #17300 (Moria SA, Antony, France).
Additionally, two clear cornea paracenteses were performed, a 1-mm at
the 6th hour for a Moria coaxial microforceps forceps insertion and a
1-mm at the 3rd hour for the anterior chamber maintainer insertion and
infusion. The DSEAK procedure was otherwise standard, to include scoring
the host Descemet’s with a reverse Sinskey hook, removal of the central
hosts Descemet’s membrane, and bi-manual pull of the DSEAK graft
through the Busin glide spatula with forceps placed through the anterior
chamber. Following the graft lenticule insertion in the anterior
chamber, the anterior chamber maintainer infusion was restarted and the
graft was unfolded. Last, a large air bubble was introduced in the
anterior chamber in order to secure superior tamponade of the graft,
against the host cornea. Following this last step, the two groups had
different completion.
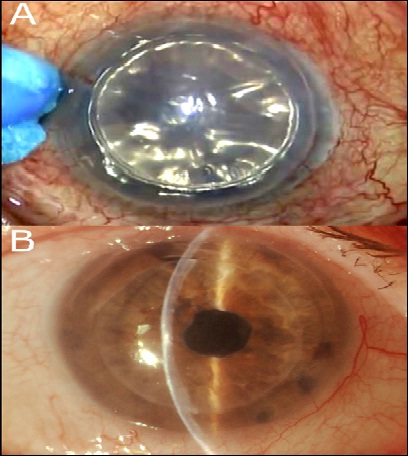
In group-A, the tissue adhesive (ReSure Adherent
Ocular Bandage, Ocular Therapeutix, Inc., Bedford, MA) was mixed on the
operating instrument stand, and applied in liquid form (painted) on the
main incision borders with a special spear sponge following the
absorption of any redundant surface fluid (Figure 1A). Within 3 to 5
seconds, the material was stable in soft form and any excess over the
peripheral conjunctiva was removed with a dry spear sponge. The eye was
observed for an additional 45 minutes for graft stability while the
anterior chamber was filled with at least 75% with air and air
tamponade, only the ‘matress’ 10-0 polypropylene sutures employing the
CS 160-6 needle (Ethilon, Ethicon Inc, Somerville, NJ) were placed to
secure the main incision. The sutures were removed typically at the
one-month visit; in all cases the sutures had been removed well prior to
the threemonth scheduled visit.
Data Collection and Analysis
All patients had been evaluated pre-operatively and
at least one-year post-operatively for best-spectacle distance corrected
visual acuity (CDVA) reported decimally, spherical and cylindrical
error reported in diopters (D), Intraocular pressure (IOP) reported in
mmHg, and endothelial cell density (ECC) reported in cells/mm2. In the
case of pre-operative ECC, the data from the cornea bank certificates
were used, while post-operatively, ECC was measured by specular
microscopy (FA-3709, Konan Medical, Irvine, CA). Slit-lamp evaluation
was also part of the complete ophthalmological evaluation performed.
Figure 1B illustrates an example of slit lamp imaging from a sutureless
DSEAK with the use of tissue adhesive, 1 week post-operatively.
Spherical and cylindrical error corresponds to the
refraction for which the CDVA was reported, and was based on phoropter
manifest refraction examination. IOP values were not adjusted for
corneal thickness changes. In addition, qualitative evaluation by means
of corneal tomography (Pentacam, Oculus Optikgeräte GmbH, Wetzlar,
Germany) and anterior-segment as well as retinal optical coherence
tomography imaging (RtVue-100, Optovue, Fremont, CA) were part of the
standard protocol performed during all visits [10]. Figure 2A presents
an example of OCT imaging performed on DSAEK case two years
postoperatively. Whenever possible, high-frequency scanning ultrasound
imaging (Artemis ii+ superior, Artemis Medical Technologies Inc.,
Vancouver, British Columbia, Canada) was also employed for the imaging
of the anterior segment. An example of Artemis cross-sectional imaging
is illustrated in Figure 2B. Due to the nature of the condition,
post-operative recovery was followed for at least once a year past their
12-month visit in all cases.
Results
The subject age in group-A (nA=15, 5 male and 10
female, 8 OD and 7 OS), at the time of the operation was 72.94±15.59 (36
to 90) years, while in group-B (nB=20, 6 male and 14 female, 9 OD and
11 OS) was 69.57±11.9 (50 to 88) years.
No case from group-A required any additional intra
operative air insertion following the tissue adhesive application and no
case required additional intra operative surgical manipulation for
further graft centration. No case required post-operative rebubbling or
had any graft rejection incidence.
In group-B, 18/20 cases required intra-operative
supplemental air insertion (re-bubbling), and four of those,
intra-operative graft repositioning. Additionally, five cases required
post-operative re-bubbling (on average 5.7 months post-operatively), and
one case lead to graft rejection, followed by penetrating keratoplasty 9
months from the initial DSAEK operation. The cases from group-B with
post-operative rebubbling
or graft failure were excluded from the subsequent data analysis,
leaving thus 14 cases whose refractive data are reported in this study,
of which 4 were female and 11 male; 7 belonged to right eyes (OD) and 8
to left eyes (OS). Average follow-up time for all cases was 10.5±8.5 (8
to 29) months.
In group-A preoperative CDVA was 0.14±0.17 (0.01 to
0.60) decimal, spherical error was -1.05±3.30 (-9.50 to +4.00) D,
cylinder was -3.75±2.05 (-10.50 to -0.50) D, IOP was 17.25±5.60 (10 to
29) mmHg, and graft/donor ECC was 2,567±310 (1,790 to 2,935) cells/mm2.
In group-B pre-operative CDVA was 0.15±0.16 (0.01 to
0.50) decimal, spherical error was -0.94±3.60 (-12.50 to +5.00) D,
cylinder was -3.24±2.44 (-10.00 to -0.25) D, IOP was 18.83±6.02 (8 to
32) mmHg. Graft (donor) ECC was 2,440±532 (1,635 to 2,850) cells/mm2.
Table 1 summarizes the pre-operative as well as the
3-month and 12-month refractive and corneal data pertaining the two
groups of study. The two groups were matched on all aspects of the
parameters involved in the study (age, gender laterality, eye
laterality, visual acuity, sphero cylindrical error, IOP and graft ECC),
as none of the paired-test p-values was less than 0.05.
The improvement in visual acuity within the same
groups had a noted and statistically significant improvement at the
3-month interval (Δ = +0.22 and +0.10 for group -A and –B, respectively)
as well as at the 12-month interval (Δ = +0.31 and +0.25). IOP was
increased (Δ = +5.51 and +4.65 mmHg for group-A and –B, respectively) at
3-months as well as at 12-months (Δ = +5.77 and +5.22 mmHg). The
increase in IOP between the two groups was rather similar, and not
statistically significant. ECC change at 12-months of -16% was noted in
group-A vs. -21% in group-B (statistically significant difference
between the two groups, p =0.03).
Figure 3 illustrates the sphero cylindrical error
(sphere and cylinder) pre-operatively, as well as 3-months and at
12-months
post-operatively. The spherical error indicated a hyperopic shift of
+1.97 D in group-A and +1.79 D in group-B (not statistically significant
difference between the two groups). Cylinder was improved (reduced), by
2.10 D in group-A, and by 0.81 D in group-B. The difference of cylinder
improvement between the two groups was statistically significant (p
=0.012).
Discussion
Management of surgical cornea incisions with nylon
sutures may have been the acceptable standard in the past, but there are
a number of complications associated with this technique [11]. Induction
of astigmatism, potential to fluid ingress and egress from the ocular
surface,[12] endophthalmitis, [13] and increase of surgical time and
patient discomfort are some that may be listed. There is, therefore,
interest in an adhesive to replace and/or supplement sutures in the
repair of corneal wounds and improve corneal incision sealing [14]. The
material must
be biocompatible, self-dissolving, and safe (eg without affecting visual
function).
Cyanoacrylate, a biocompatible material, has long
been employed in surgical incisions [15]. The latest cyanoacrylate
adhesive for medical use, FDA-approved in 2002, was n-butyl-2-
cyanoacrylate (Indermil®, Vygon-Ecouen, Lansdale, PA). It has been used
to close small skin wounds in pediatric patients, with successful
results [16]. Applications in ocular surgery have also been reported
[17,18].
Polyethylene glycol (PEG) polymers are also among the
materials considered for corneal wound sealing [19]. Biocompatible PEG
polymers form the same type of hydrogel used in contact lenses in
wetting agent applications [20]. The liquid hydrogel compound is
administered (painted) over the wound, and polymerizes fast (in
approximately half a minute) into a soft form that adheres to the ocular
surface, forming a bandage that leads to a watertight seal. Two such
ocular tissue adhesive products are the ReSure (Ocular Therapeutix,
Inc., Bedford, MA) [21] and the OcuSeal (BD Medical, Waltham, MA) [22].
Ocular tissue adhesives have been evaluated for their
applicability in limbal-conjunctival wound after fornix-based
trabeculectomy [23] and cataract surgery [24]. To the best of our
knowledge there is no report in the peer-reviewed literature on the
topic of ocular tissue adhesives in DSAEK.
The present work is to the best of our knowledge the
first investigation that presents the clinical applicability of
employment of ocular tissue adhesive in DSAEK. We evaluated
comparatively two matched groups of study over a long followup period.
The differences in regard to far fewer cases needing intra-operative
re-bubbling have been compelling. Postoperatively, similar visual acuity
and IOP changes are noted. CDVA was improved in both groups, while also
IOP increase has been noted; the latter can be explained by the thicker
cornea (as a result of the DSAEK procedure) and the known dependence of
IOP readings on central corneal thickness [25].
We noted a statistically significant ECC loss in both
groups (by -16% in group-A, and by -21% in group-B). These data are in
accordance with published results in the literature. For example, ECC
loss of -19% has been reported in DSAEK cases [26]. We note, however,
that the ocular adhesive group-A appears to perform better in this
aspect (p =0.045 between the two groups).
Regarding the refractive data, both groups indicate a
significant hyperopic shift. The average increase in sphere was more
than +2.50 D at the 3-month interval and near +2.00 D at the 12-month
interval. This hyperopic shift has been modeled, and the average
predicted hyperopic shift in the overall power of the eye was calculated
to be +0.83 D [27]. It is explained by the fact that the graft is
thinner centrally, as a result of the microkeratome pass creation
procedure over the donor cornea, and the known increased corneal
thickness peripherally. It appears that our technique introduces more,
but predictable hyperopic shift. As a result the graft is thinner
centrally and thicker peripherally, the ‘new’ posterior corneal surface
has a smaller radius of curvature; our calculations based on application
of the Gullstrand’s formula indicate that for approximately 1 mm change
in the posterior curvature (e.g from 6.8 to 5.8 mm) there is a
corresponding 1.00 D change in the posterior corneal refractive power
(e.g. from -5.88 D to -6.90), resulting thus in 1.00 D of hyperopic
shift.
A noted improvement in cylinder was noted in both
groups, with the ocular adhesive group-B appears to perform better in
this aspect (p =0.032). The sutureless group-A had 12-month improvement
in cylinder by 2.1 D, while the suture group-B by 0.8 D. This may be
explained by the reduced effect on cornea distortion by the ocular
adhesive. Considering the nonsymmetrical nature of the suture placement
in the traditional wound sealing, the noted improvement in
surgically-induced astigmatism in the sutureless group-A may offer
perhaps the clinical advantage over the traditionally applied technique.
Application of a material that seals wounds safely,
effectively, and comfortably enable better suture placement and possibly
improve corneal surgical outcomes. This procedure enables better suture
placement and provides the benefit of the reduction of wound leak
during suturing and possible graft slippage. This procedure may also be
applied to DMEK cases. Further studies involving the clinical impact of
the use of these new polymer corneal sealants may be warranted.
Conclusions
This novel tissue adhes ive may be a valuable adjunct
in sutureless DSEAK clear cornea surgery in enhancing intra operative
anterior chamber stability and possibly offering more secure wound
closure.
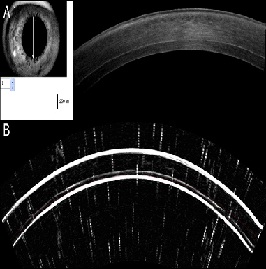
Figure 2: Cross-sectional imaging of a DSAEK case two years postoperatively: top, utilizing anterior-segment OCT, and bottom, utilizing high-frequency scanning ultrasound.
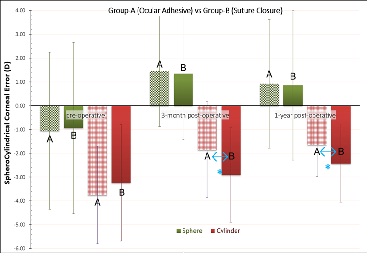
Figure 3:
Comparative spherocylindrical error between the two groups,
pre-operative, 3-months and 1-year post-operatively. *indicates
statistically significant difference between the two groups.
For more articles in JOJ Ophthalmology please click on:
https://juniperpublishers.com/jojo/index.php
https://juniperpublishers.com/jojo/index.php
No comments:
Post a Comment