JUNIPER
PUBLISHERS- JOJ Ophthalmology
Abstract
Purpose: To report novel optical coherence tomography findings in a case of anti-α-enolasecancer associated retinopathy.
Observations: An elderly female presented with
bilateral decreased vision and a recent diagnosis of ovarian carcinoma.
Optical coherence tomography demonstrated bilateral loss of outer
retinal structures and macular edema. Serum testing found antibodies
against α-enolase and 82-84kDa proteins. Outer retinal structures showed
recovery, macular edema resolved and repeat anti-retinalantibody
testing became negative following cancer therapy and topical
difluprednate treatment.
Conclusion and importance: Cancer associated
retinopathy is a paraneoplastic disease that results in damage to
retinal structures through an autoimmune response. The damage is
generally considered to be irreversible however, in rare cases, such as
observed here, retinal structures may demonstrate recovery after
treatment.
Keywords: Cancer associated retinopathy; Optical coherence tomographyIntroduction
Cancer associated retinopathy (CAR) is a
paraneoplastic disease in which retinal degeneration occurs as an immune
response to cancer antigens sharing homology with endogenous retinal
proteins [1].
Past studies have found various retinal proteins to be antigenic, which
include recoverin, α-enolase, arrestin, and transducin [https://www.ncbi.nlm.nih.gov/pubmed/245316532].
The inhibition of enolase, a glycolytic enzyme, results in metabolic
disruption of retinal cells and the induction of apoptosis [3].
Anti-α-enolase autoantibodies are capable of accessing tissue and
targeting ganglion cells, Muller cells, and photoreceptors. It is
believed that death of retinal cells is an irreversible process. We
report a patient with gynecologicalCAR who experienced objective
improvement in photoreceptor architecture following treatment of her
underlying malignancy.
Case Report
An 80 year Hispanic female with a history of chronic,
bilateral Vogt-Koyanagi-Harada associated uveitis presented to the
Casey Eye Institute Uveitis Clinic for a routine follow up visit. At
that time, she reported a newdiagnosis of ovarian carcinoma and had
started her first chemotherapy session consisting of carboplatin and
paclitaxel. Due to severe aortic stenosis, the patient was not a
candidate for surgical intervention. Her vision was 20/30 bilaterally
without evidence of active uveitis. Four months later she returned with a
bilateral decrease in vision to 20/50. The patient underwent imaging
with macular volume scans centered on the fovea (Heidelberg Spectralis
spectral domain ocular coherence tomography (OCT) with eye tracking
software, Heidelberg, Germany) that demonstrated a disrupted inner
segment/outer segment junction (ISOS) and cystoid macular edema (CME)
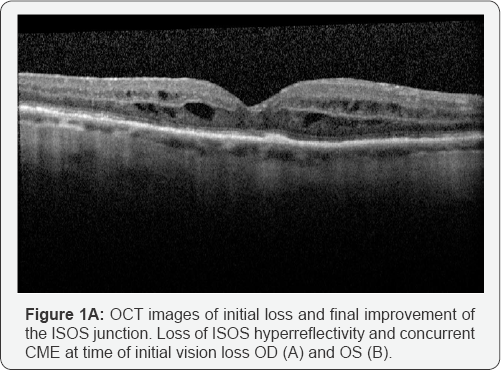
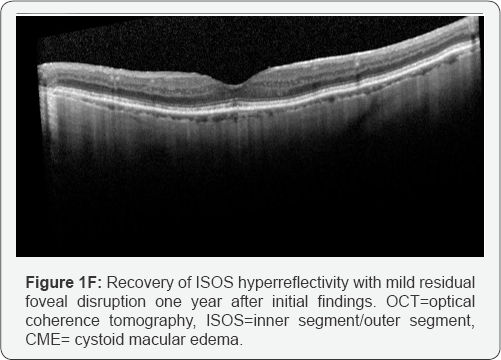
bilaterally (Figure 1A & 1B).
Clinical and OCT findings were suspicious for CAR and anti-retinal
antibody testing was pursued. The patient declined local or systemic
immunosuppression specifically for her ophthalmic diseaseand continued
to undergo planned chemotherapy. One month later, her vision had dropped
to 20/50OD and 20/100OS. Repeat OCT mapped to the original images
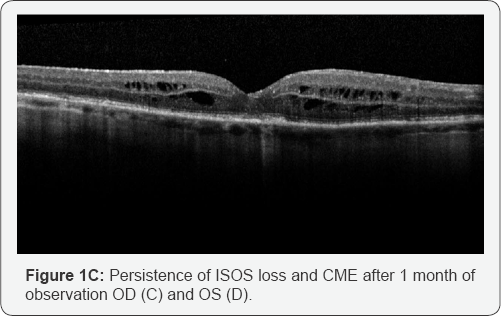
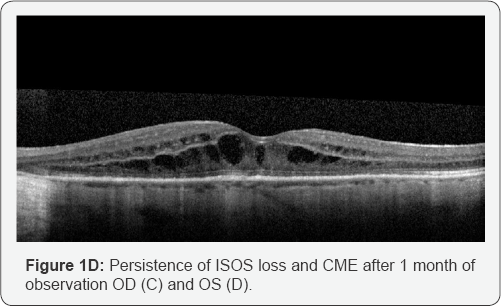
continued to demonstrate loss of the ISOS junction and CME in both eyes (Figure 1C & 1D).
Serum tested for the presence of anti-retinal autoantibodies showed
antibodies against α-enolase and 82-84kDa proteins. Immunohistochemistry
of the patient’s serum showed positive staining of the photoreceptor
cell layer in human retina. The patient continued to decline periocular
injection or systemic immunosuppression and was prescribed difluprednate
drops three times daily. Two months later, there was partial return to
normal reflectivity of the IS/OS junction on OCT and the CME had
improved. Six months later, there was resolved CME on OCT and the IS/OS
reflectivity returned to near normal in the subfoveal region. At this
time, the vision was 20/40 bilaterally. The patient was instructed to
stop difluprednate drops. Over the following six months, the patient’s
visual acuity stabilized at 20/60OD and 20/50OS. There was no recurrence
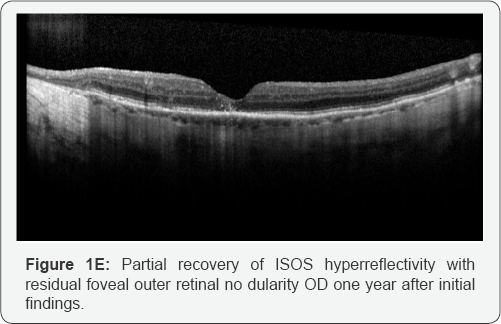
of CME and the OCT showed normalized IS/OS reflectivity except in the
fovea where there was a stable elevated outer retinal lesion OD and
near-normalized ISOS reflectivity in the left macula except in the fovea
(Figure 1E & 1F). Serum was negative for anti-retinal autoantibodies on repeat testing.

Discussion
Anti-retinal autoantibodies can be detected in both
retinopathy patients and healthy individuals. Individuals with
gynecological CAR have a higher proportion of seropositivity than normal
individuals [4,5].
Our patient became symptomatic after diagnosis of ovarian cancerand
initiation of chemotherapy treatment. Autoantibodies may be present
before the diagnosis of cancer, but it is not until they breach the
blood retinal barrier that symptoms become evident [4].
Although the presence of anti-retinal autoantibodies can occur in
normal patients, high antibody titers are a better indicator of
retinopathy [5].
Anti-enolase autoantibodies affect the catalytic activity of the enzyme
thus depleting glycolytic ATP, increasing levels of intracellular
calcium which then induces mitochondrial-mediated apoptosis by the
activation of its key elements [3].
The loss of outer retinal structures and retinal
atrophy observed in autoimmune retinopathy arefrequently thought to be
irreversible [5,6].
Partial recovery of SD-OCT outer retinal changes in a patient with CAR
after treatment with rituximab has been reported, which suggests that
therapy targeting B cells and consequently reducing production of
anti-retinal antibodies may be beneficial [7].
Our patient showed improvement of the reflectivity of the photoreceptor
IS/OS junctiondespite only local therapy with difluprednate, which was
started to treat CME and reduce local inflammatory damage, but unlikely
to significantly affect autoantibody production. We hypothesize that the
prompt initiation of chemotherapy may have contributed to the patient’s
improvement by possibly decreasing tumor expression of enolase and
diminishing the production of anti-retinal autoantibodies or that
chemotherapeutic treatment non-specifically immunosuppressed antibody
production.The recovery of outer retinal structures in the present case
corresponded to anti-retinal antibodies no longer being detected in the
patient’s serum, supporting a pathologic role for autoantibodies in our
patient.Treatments that may limit the production of anti-retinal
antibodies such as rituximab should continue to be studied for efficacy
in CAR.
The history of prior uveitis is a potential
confounder to our findings; however, the patient did not demonstrate
active inflammation during this follow up period. The presence of CME
may also confound the ability to image the outer retina; however, we
observed patchiness of the ISOS junction outside of regions of retinal
edema indicating the outer retinal changes were not a sequel of CME
alone. CME is not a common manifestation of CAR, more frequently
observed in non-paraneoplastic autoimmune retinopathy [8], but previous case reports of CAR-related CME suggest it is responsive to steroids, as was observed in our patient [9].
Unfortunately, the patient declined additional objective testing
(visual fields, electroretinography), which would have allowed further
clinical correlation.
We report a patient with CAR who experienced
objective improvement in photoreceptor architecture following treatment
of her underlying malignancy, a novel observation previously reported
only following rituximab therapy. We also note the successful treatment
of CAR-associated CME with topical difluprednate, suggesting an
alternative therapy to previously reported treatments with periocular or
intravitreal steroids.
Conclusion
Damage to retinal structures from CAR can be
objectively captured by OCT and may demonstrate recovery after treatment
in rare cases.
Patient Consent
Consent to publish the case report was not obtained.
This report does not contain any personal information that could lead to
the identification of the patient.
Funding
P30 EY010572 from the National Institutes of Health
(Bethesda, MD), unrestricted departmental funding to the Casey Eye
Institute from Research to Prevent Blindness (New York, NY).
Conflict of Interest
JTR, PL, GA. The following authors have no financial
disclosures FJI, LJK, SSS, MS, KB. All authors attest that they meet the
current ICMJE criteria for Authorship.
For more articles in JOJ Ophthalmology (JOJO) please click on: https://juniperpublishers.com/jojo/index.php
No comments:
Post a Comment