Juniper
Publishers- JOJ Ophthalmology
The endogenously generated small nonapeptide
bradykinin (BK) is often associated with inflammation, edema and pain
amongst many other functions and pathologies. However, the latter
aspects pertain to locally produced BK from circulating plasma precursor
polypeptide kinninogen (KNGN). Recent work in ocular tissues and cells
have a revealed a novel local synthesis of BK and other kinins from
tissue-derived KNGN via action of kallikrien enzymes, quite independent
from the blood-derived polypeptide. Furthermore, the whole kininergic
system machinery including local KNGN, kallikrein enzymes to generate
kinins from KNGN, two sub-types of kinin receptors (Bj-and B2-receptors),
and the complete signal transduction pathways coupled to these
receptors have been mapped out. Additional work has highlighted a number
of downstream signaling and other biological responses that ensue
following activation of Ba- and B2 receptors in
human ocular cells and tissues. One key aspect to be discussed in detail
in this review is the novel finding that BK, peptidergic BK analogs,
and especially non-peptide mimetics of kinins (e.g. FR-199097; BKA78),
profoundly lower and control intraocular pressure in a number of
species, including ocular hypertensive (OHT) monkeys. These novel
observations strongly suggest that kinin agonists represent a novel
class of ocular hypotensive agents that could be of immense value in
treating OHT associated with primary open-angle glaucoma (POAG) and
perhaps other forms of glaucoma.
At a simplistic level, our knowledge has advanced to a
point where it is now clear that elevated intraocular pressure (IOP)
results from a fundamental imbalance between the generation of aqueous
humor by the ciliary body and its efflux from the anterior chamber of
the eye via one of two pathways [
1-
3].
The most physiologically relevant mechanism of AQH drainage involves
the IOP-dependent outflow via the trabecular mesh work (TM) and
Schlemm's canal (SC) route [
1-
3]. The lesser utilized pathways under normal conditions are the uveoscleral [
1-
3] and ocular lymphatic [
4]
pathways, but the latter can be engaged by certain drugs such as
FP-class prostaglandin analogs (FPGAs) like latanoprost and tafluprost [
1-
6].
The chronically increased IOP, a condition generally termed as ocular
hypertension (OHT), caused by blockage of the AQH drainage TM/SC
pathways [
7]
during the aging process or due to ocular inflammation and deposition
of various debris, along with apoptotic death of retinal ganglion cells.
can lead to a clinically defined disease called glaucoma [
8-
10].
This high
IOP distressfully distends and traumatizes the whole Whilst, glaucoma is
painless and otherwise asymptomatic globe and initiates the death of
RGCs and/or breakage of RGC axons at the back of the eye. These elements
then cause a retrograde demise of the RGCs leading to severing of nerve
fibers connecting the retina to the brain [
11-
13].
Many deleterious neurotoxic elements (e.g. high levels of extruded
glutamate, endothelin, inflammatory cyto-and chemo-kines, noxious gases
(e.g. nitric oxide; hydrogen sulfide)) and proteolytic enzymes (e.g.
caspases and matrix metalloproteinases) released by activated
macrophages [
12-
19],
injured RGCs and interneurons are the culprits responsible for such
neurotoxicity/chemically-induced axotomy of the RGCs. Hypoxia and
ischemia [
20,
21]
are also involved in the initiation phase of vascular
dysfunction-induced death of RGCs since the thinning of the optic nerve
at the level of the optic nerve head (ONH) forces the retinal blood
vessels attached to the optic nerve to bend thereby restricting blood
supply to the retina. While this progressive loss of RGCs occurs over
several decades, if OHT is not treated to reduce the IOP
the resulting glaucomatous optic neuropathy causes severe visual
impairment, reduction of visual acuity and visual field and eventually
results in irreversible blindness. Although many forms of glaucoma exist
(e.g. open-angle glaucoma; closed-angle glaucoma; exfoliation glaucoma;
myopic glaucoma) [
8,
9,
22-
26], the most prevalent is primary open-angle glaucoma (POAG) [
26-
28].
Behind cataracts, POAG is the second leading cause of blindness
afflicting several millions of patients. It is estimated that by 2020,
the global glaucoma-related blindness will reach ~80 million [
26-
28]. Elevated IOP and advancing age are the two major risk factors associated with POAG even though genetic factors [
26] and race (especially African and Asian heritage) [
29], myopia, diabetes and oxidative stress [
30-
36] and various vascular irregularities and dysfunctions [
20,
22], and intracranial cerebrospinal pressure [
37,
38]
have been also
linked to the development of POAG. The seriousness of POAG is often
underestimated since it causes no overt discomfort or pain to the
patient and insidiously progresses unnoticed over time. Considerable
damage to the retina [
33-
36] and optic nerve and optic nerve head (ONH) [
14,
29,
39-
41]
continues unyieldingly leading to scotomatous damage that manifests as
loss of peripheral vision followed by a "tunnel vision" syndrome,
thereby finally signaling the demise of ~40% of the original million
RGCsof the patient and equivalent loss of connections to the brain and
visual cortex [
8,
9,
42-
45]. Those lost or dying RGCs cannot be resuscitated and their axonal connections revived [
46-
48],
and if left untreated the glaucomatous optic neuropathy due to OHT and
oxidative /neurotoxic elements would claim the remaining RGCs causing
total blindness [
49-
53].
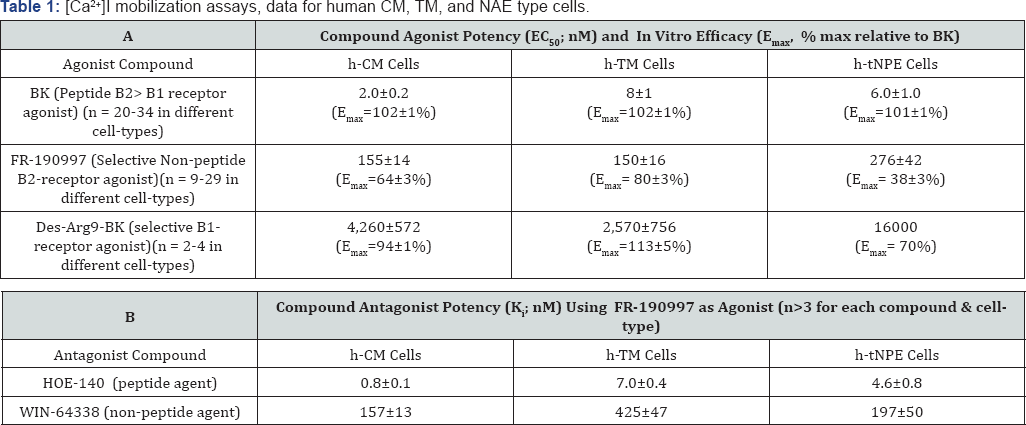
Data are mean±SEM uses a [Ca
2+]I
mobilization assay in cells derived from several different human donors'
eyes. h-tNPE cells are SV40-virus- immortalized human non-pigmented
ciliary epithelial cells derived from human ciliary epithelium that
respond just like normal primary NPE cells. [
145,
146,
157]
A number of treatment options have been developed to deal with POAG-associated OHT including ocular hypotensive medications [
1-
3,
54-
56], laser therapy and surgical interventions [
57-
64].
Unfortunately, as is the case with most drugs and surgical procedures,
these treatment modalities have numerous side-effects (e.g. Burning and
stinging, foreign- body sensation, brow-ache, ocular surface dryness,
pulmonary hypertension and bradycardia, etc.), and adverse complications
associated with them [1-3,54,55]. Additionally, poor compliance [
65]
and adherence to prescribed topical ocularly administrated medications
by the OHT POAG patients (due to forget fulness, poor dexterity,lack of
symptomatic pain or other cues due to OHT, poor understanding of the
treatment regimen, and perhaps due distrust, etc.) contributes to the
progression of the disease process. Similarly, laser therapies, although
effective at the beginning, lose their efficacy over time [
57-
62].
Reports have surfaced that indicate that POAG/ OHT is only controlled
in ~50% of patients who received laser treatment, and indeed due to
scarring,the procedure often needs repeating within one-five years [
57-
64].
Indeed,
such lasering and filtration procedures also have certain risks of
complications and adverse events associated with them. Thus, there
remains a continued unmet medical need to discover new and improved eye
drop- medications and other novel surgical techniques to help the
OHT/POAG patients mitigate and treat their underlying glaucomatous optic
neuropathy caused by elevated IOP. To this end, a better understanding
of the many complex pathways involved in AQH dynamics [
4,
7-
10] has culminated in the discovery and development of many novel targets and ligands [
13,
54,
55]
that can modulate IOP via these targets to accomplish a level of
homeostasis of AQH production and drainage. In order to address poor
patient compliance, a number of innovations leading to sustained
drug-release devices (e.g. implants, punctal plugs or contact lenses) [
60-
64,
66-
70]
have been developed such that the patient need not remember to
self-administer the medication. Likewise, a revolutionary set of novel
surgical interventions [
57-
64] with much reduced surgical time and effort required and minimal adverse events and complications are becoming available [
57-
64].
These include the following: non-penetrating glaucoma surgery (NPGS),
non-invasive glaucoma procedure, minimally invasive micro sclerostomy,
blebless ab externo glaucoma surgery, ab externo bleb surgery, and the
elegant minimally-invasive glaucoma surgery (MIGS) [
60-
64]
that involve insertions of tiny drainage devices into the anterior
chamber of the eye that appear to be highly effective in decreasing IOP [
62-
64].
Time will tell if indeed these innovations become mainstay treatment
options for POAG/OHT patients in the near future. Regardless, however,
the ordinary patient who is unable to afford the latter surgical
procedures and devices, and those patients who are refractory to or
cannot tolerate existing medications, will still require new topically
administered drugs to lower and control the IOP in order to preserve
their vision.
One of the earliest pharmacological agents to be used
to lower elevated eye pressure is a muscarinic receptor agonist,
pilocarpine [
1-
3].
It just happened that it promoted egress of AQH through the TM, hence
pilocarpine became the first known conventional outflow promoter. Since
it strongly constricted the pupil, made accommodation difficult and
painful due to brow- ache, and needed to be administered up to 4-times a
day, newer drugs with higher efficacy and lesser side-effects were
sought and discovered over the next few decades. These included carbonic
anhydrase inhibitors such as dorzolamide and brinzolamide,
beta-adrenergic antagonists (e.g. timolol; betaxolol) and alpha-
adrenoceptor agonists such as brimonidine and para-amino- clonidine
[1-3,54,55]. Whilst these drugs lowered IOP well, they required at least
twice daily dosing and they primarily inhibited the production of AQH
by the ciliary processes of the ciliary body. Eventhough compliance
increased and a greater efficacy was achieved, these agents had their
own short-comings in terms of side-effects including ocular surface
irritation (hyperemia), burning and stinging,allergy and drowsiness
(a-agonists), and some pulmonary and cardiac insufficiency (with
p-blockers) [
13,
55]
Furthermore, we have learnt that AQH constituents serve important
nutritional needs of the tissues inside the anterior chamber of the eye [
10],
and thus reducing its availability negatively impact the ciliary body,
lens, corneal endothelial cells, and may in fact damage the TM and SC
endothelial cells. Thus, a major breakthrough in OHT / POAG treatment
occurred in the mid-90s when FP-receptor-selective prostaglandin (PG)
agonists (PGAs) (e.g. latanoprost; travoprost; bimatoprost; tafluprost;
unoprostone isopropyl ester) [
3,
5,
6,
71-
73]
were discovered and introduced into ocular clinical medicine. These PG
drugs revolutionized the POAG/OHT treatment paradigm since they required
once-daily ocular administration (before bed-time) and were much more
potent and efficacious than the existing medications since they created
new drainage pathways across the ciliary muscle and sclera (uveoscleral
pathway) to help drain the AQH [
1-
5].
Nevertheless, these novel PGAs had some significant side-effects that
included hyperemia, darkening of the iris color and increased
pigmentation of the periorbital skin, growth of eye-lashes, deepening of
the eye orbit, and in some cases cystoid macular edema [
1-
5,
71-
74].
Additionally, some OHT/POAG patients were quite refractory to the PGA
drugs such that they required multiple drugs to control their IOPs. Not
surprisingly, a multitude of fixed-dose combination products [
4,
75]
containing different dual combinations of various ocular hypotensive
drugs (and even a triple combination product) have now become available.
However, due to the inherent genetic and biological variation in
responses of patients to the different classes of IOP-lowering
medications and their relative susceptibility to the side-effects of the
drugs, there still remains a great need to hunt for and discover new
drugs that are more effective, longer acting, efficacious in majority of
OHT patients, and that induce fewer and milder off-target side-effects,
thus having a greater therapeutic index than the existing drugs [
67,
76,
77] (
Table 3).
The potential additivity of new pharmacological
agents to PGAs in the treatment of OHT and POAG has spurred the recent
surge in research for novel agents exhibiting IOP-lowering properties.
The realization that POAG is not only caused by elevated IOP since
patients with normal IOPs still lose vision [
7880], but perhaps is a reflection of enhanced RGC susceptibility to oxidative stress [7-9,12,32-36,42] and apoptotic process [
15,
47,
81-
90],
has stimulated a renewed interest in finding drugs that have dual or
multiplicity of mechanisms of action, including direct potential
neuroprotective activity. The latter aspect stems from the finding that
agents like betaxolol [
91-
95] and brimonidine [
90,
96-
99], whilst lowering IOP, also upregulate the release of endogenous neurotrophins [
89]
in retinal tissues that could heal/rescue some of the RGCs that are
compromised from the elevated IOP and oxidative stress. Likewise certain
PGAs stimulate blood flow at the ONH in addition to reducing IOP [
5,
6,
100]. Additionally, as the tools to monitor IOP [
98,
99] have become more accessible at a lower cost and with a greater sensitivity, including round-the-clock monitoring of IOP [
99,
100],
and as new models of OHT/POAG are introduced using various species
[48,23-25,101-104], the potential for such innovations to enhance drug
discovery have dramatically increased in recent years. The ability to
perform AQH dynamic measurements in small laboratory animals like mice [
105], and to exploit enucleated and ex-vivo perfused bovine [
103,
106], porcine [
103] and human eye anterior segments, and even whole eye [
107],
has further accelerated the mechanistic approach to ocular drug
discovery and characterization. Accordingly, pharmacological agents that
have exhibited ocular hypotensive efficacy in some of these animal/
ex-vivo models include K+- channel openers [
108], Na+-K+ -ATPase inhibitor digoxin analogs [
109], angiotensin-II receptor antagonists [
110,
111], renin inhibitors [
112], angiotensin converting enzyme (ACE) inhibitors [
113-
116], ACE-2 activators [
117,
118], cannabinoids [
119], rho- kinase inhibitors [
120-
122], nitric oxide (NO) donors and their conjugates [
123-
127], serotonin (5-hydroxy-tryptamine (5-HT)) receptor agonists [
103,
128,
129], hydrogen sulfide donors [
130], dopamine receptor agonists [
3], melatonin receptor agonists [
13], adenosine receptor agonists and antagonists [
131], guanylate cyclase activators [
123,
132], novel EP2 receptor agonists [
133,
134], dual pharmacophoric PGs encompassing FP and EP3- receptor agonistic properties [
135,
136],
etc. The most recent unexpected discoveries of potential drug
candidates for OHT/ POAG treatment pertain to the kallikrein-kinin (KNK)
system which will be addressed in detail below (
Table 4).
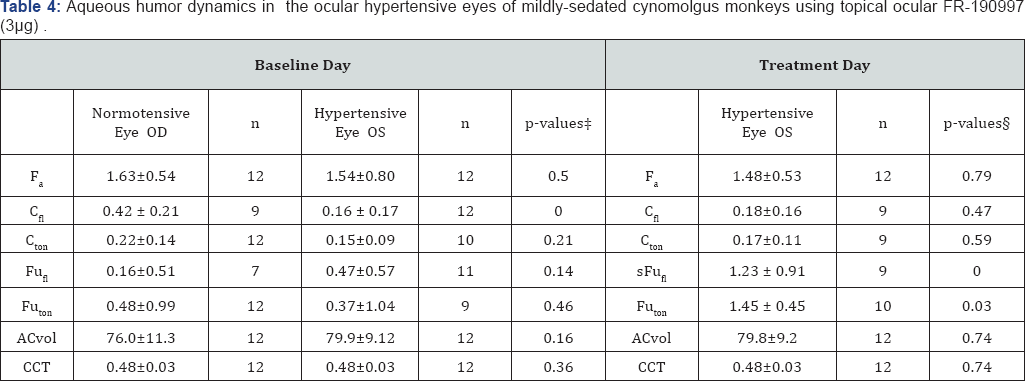
* Values are means±standard deviation. t, comparing
hypertensive with contralateral normotensive eyes; �p-values: comparing
baseline day with treatment day by Student's two-tailed paired t-test.
ACvol, anterior chamber volume, |jl; CCT, central cornea thickness, mm; Cf| fluorophotometric out flow facility, iJl/min/mmHg; Cton, tonographic outflow facility, |j1/min/mmHg; Fa, aqueous flow, |jl/min; FuFI, uveoscleral outflow calculated with Cfl, jl/min; Futon, uveoscleral outflow calculated with Cton
jl/min; Times are 30 minutes. FR-190997 (0.01%) was applied as a 30jl
drop (total dose of 3jg) to each eye of each monkey Modified from Ref
155.
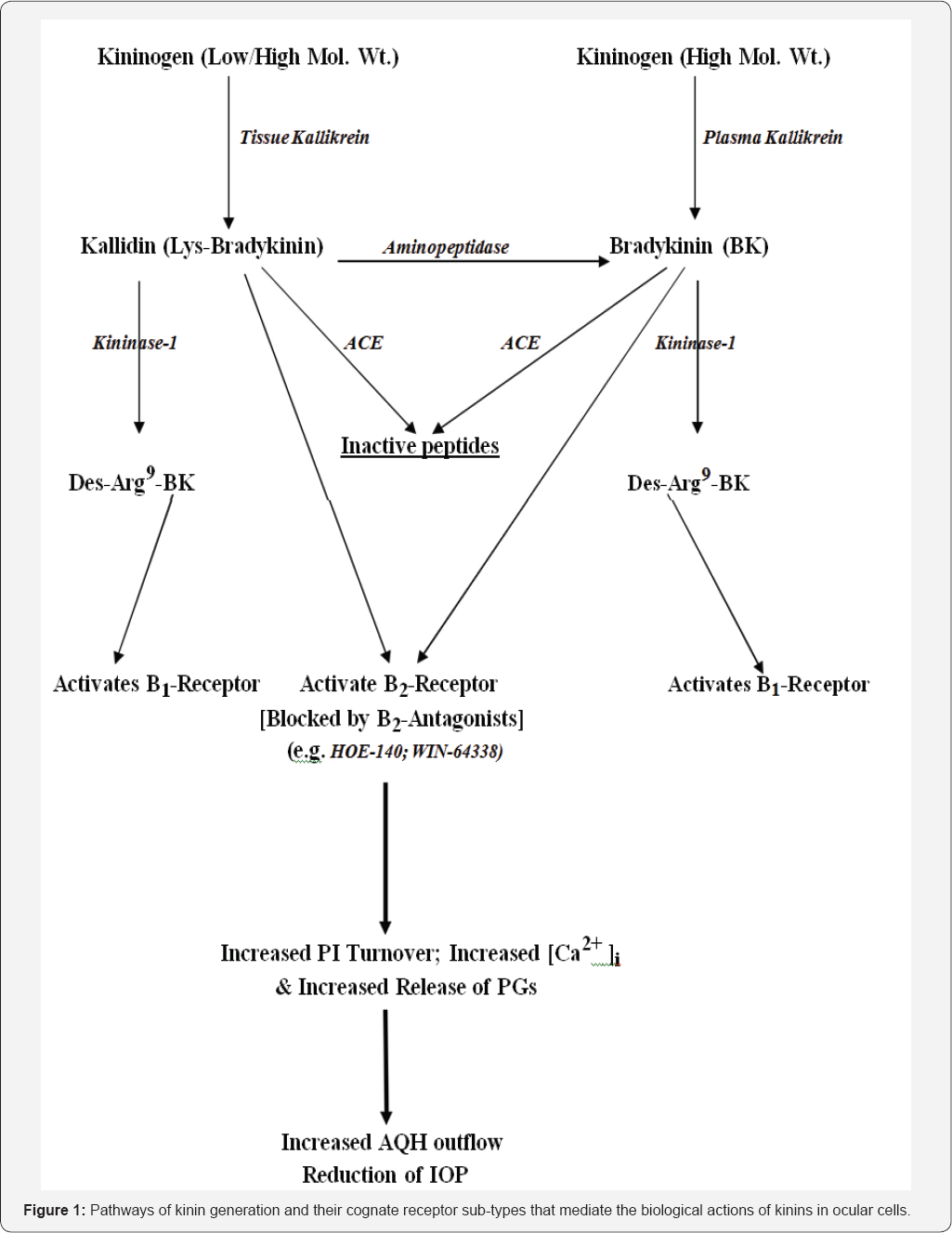
The peptide bradykinin (BK) was discovered many
decades ago and the pathway to its generation has since been fully
delineated. Today, we know that a precursor polypeptide, kinninogen
(KNGN), is cleaved by specific enzymes (kallikreins) to produce a
10-amino acid -and a nine-amino acid containing peptide, Lys-BK and BK
respectively [
137,
138]. Lys-BK is then converted to BK by an aminopeptidase, but both Lys-BK and BK act on the B
2-receptor subtype of BK receptors, which is the predominant homeostatic receptor found Under normal physiological situations [
137,
138]. Kininase-1can convert both Lys-BK and BK to an octapeptide (Des-Arg9-BK) that interacts specifically with B
1-receptor subtype of BK receptors which get upregulated during injury, trauma and other deleterious situations [
137,
138]. ACE inactivates both Lys-BK and BK to small inert peptides (
Figures 1,
2).
The notoriety surrounding BK and Lys-BK (kallidin) originates from
their ability to cause deleterious vasodilation, inflammation, pain and
cell proliferation [
137,
138].
These undesirable effects of kinins have been noted in all parts of the
body and either trigger or are manifestations of various underlying
diseases ranging from angioedema, diabetes, pulmonary and systemic
hypertension, and aneurysms and diabetic retinopathy in the central
nervous system and retina [
137-
139].
It was quite a revelation when various components of the KNK system
were found in various compartments of the eye under normal circumstances
and associated with cells and tissues of the eye [
140,
141].
Soon it became clear that BK and perhaps Lys-BK could be formed locally
by the actions of tissue-based kallikriens on tissue-derived KNGN
thereby creating a paracrine kininergic system within ocular tissues [
140,
141].
To support this notion further, both B1 and B2-receptor subtypes were
found in various ocular cells that were functionally active mediating
the actions of BK and related analogs of BK, generating intracellular
second messengers [
142145]
Figure 3 producing further downstream effects such as promoting liberation of PGs [
146,
147] and causing ocular tissue contraction/ relaxation [
148-
150],
etc. While circulating KNGN and kallikriens in ocular blood vessels do
produce BK and Lys- BK to cause the vasodilator and pro-inflammatory
effects as in the rest of the body, that system is distinctly different
from the tissue-based KNK system in the ocular systems.
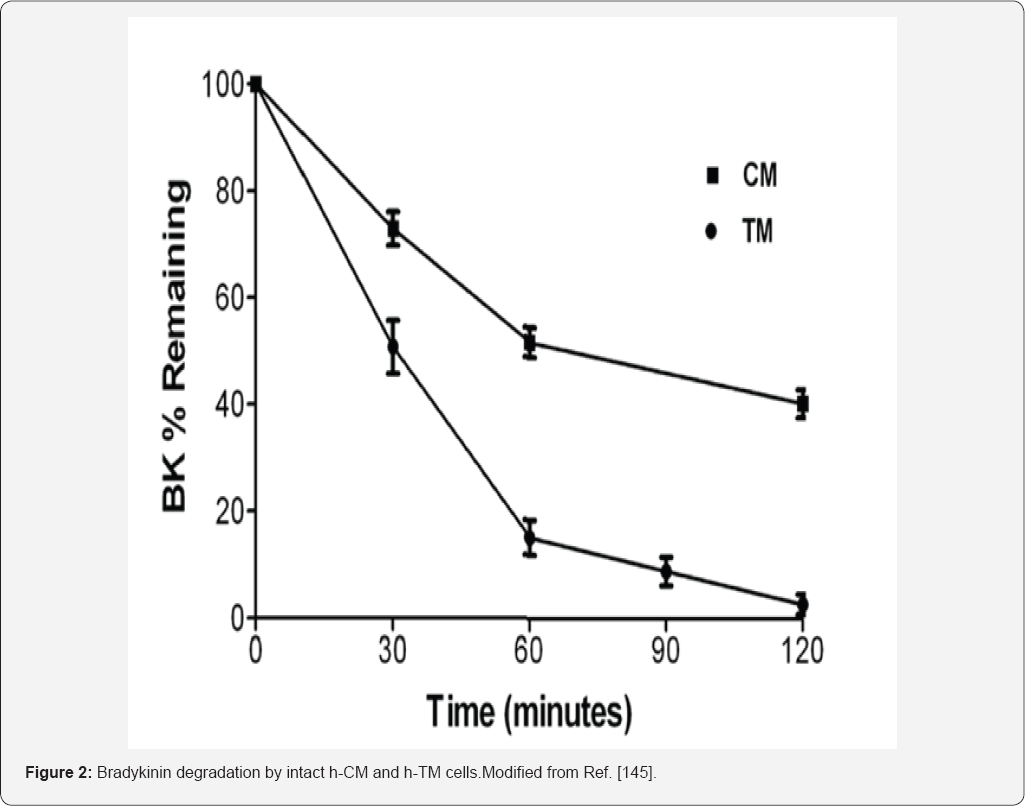



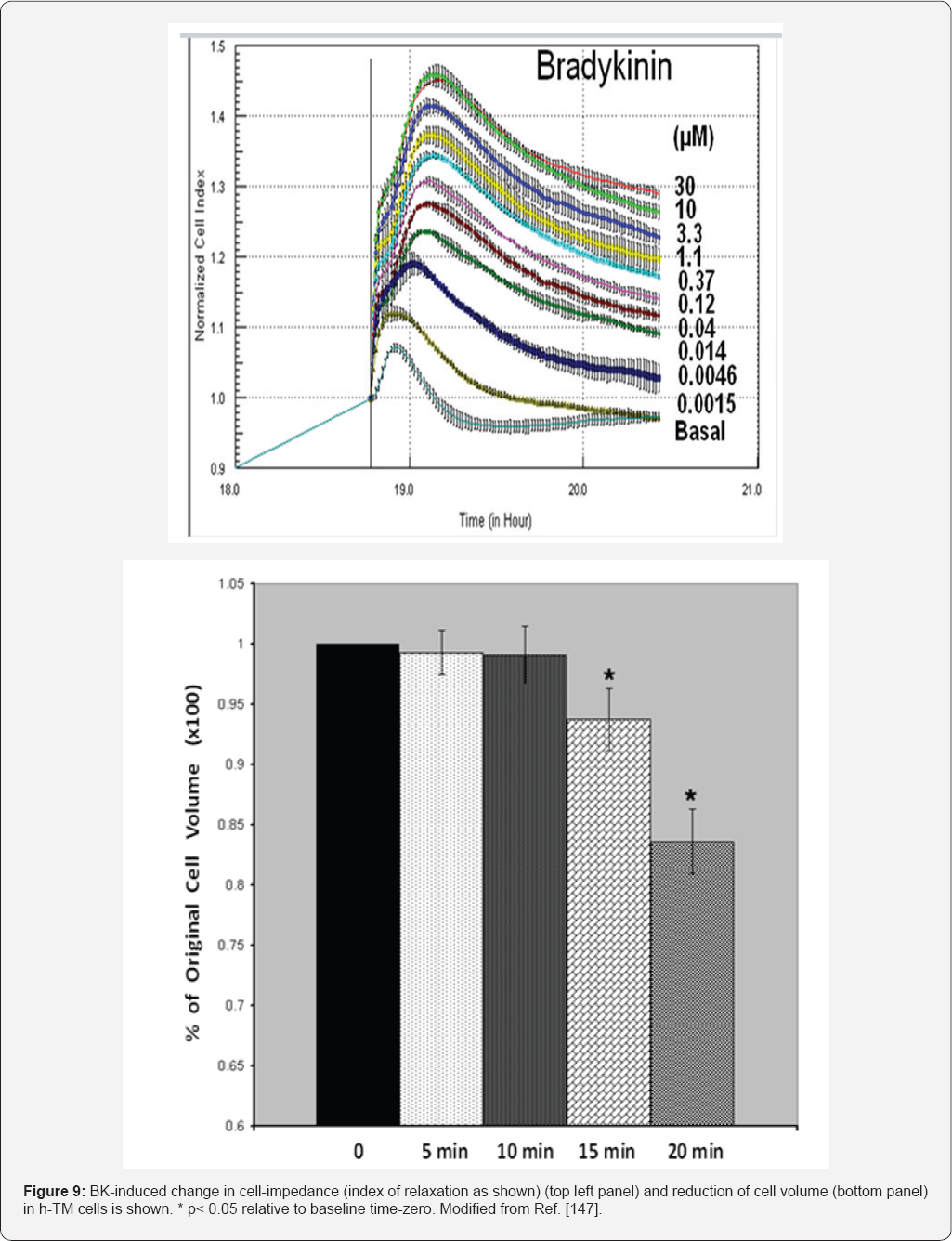
In order to ascribe a role of BK in ocular functions,
early researchers administered BK either systemically or topical
ocularly (t.o.) or via injections into the anterior chamber of the eye
of different species [
151-
154]. BK either increased or decreased IOP accompanied by local inflammation and/or miosis [
148-
155].Using ex-vivo isolated bovine [
106] and porcine [
103,
156]
eye anterior segments, perfused BK was shown to induce disparate
results, either causing decreased outflow or increasing outflow of AQH,
thereby adding to the overall confusion. The known metabolic instability
of BK in the presence of fluids or exposed tissues/cells [
145,
157],
and possible species differences potentially contributed to these
contradictory observations. The need for metabolically stabilized BK
analogs or non-peptide BK mimetics was soon realized (see below). The
rather paucity of information regarding the ocular BK receptor family in
human ocular cells and tissues was slowly overcome by research in the
early 80s-90s, including the work oflgic [
140], Ma et al. [
141], Sharif et al. [
142], Wiernas et al. [
143,
144], and by Webb et al. [
106,
145,
157].
The work of Ma et al. [
141]
using reverse transcription- polymerase chain reaction (RT-PCR) and
Southern blot analyses, and in situ hybridization to localize components
of the KNK system in human ocular tissues was affirmed using
immunohistochemistry (IHC) [
145-
147].
For the current subject matter of this review article, it was important
to demonstrate the specific distribution and localization of the B2-BK
receptor proteins. Accordingly, the presence of B2-BK receptors in human
TM [
147], ciliary muscle (CM) [
146] and non-pigmented ciliary epithelial (NPE) [
158] cells was demonstrated by IHC techniques. These are all key tissues involved in the drainage (CM and TM)
Figure 4
and production (NPE) of AQH respectively. Importantly, these IHC
observations were extended to the cynomolgus monkey anterior chamber
tissues [
146-
148]
in order to help correlate functional in vivo data (IOPlowerig; AQH
dynamics) with these in vitro observations (see ahead). Next, it was
important to determine whether the IHC of B
2-receptors bore
any linkage to functionality of these proteins in the respective human
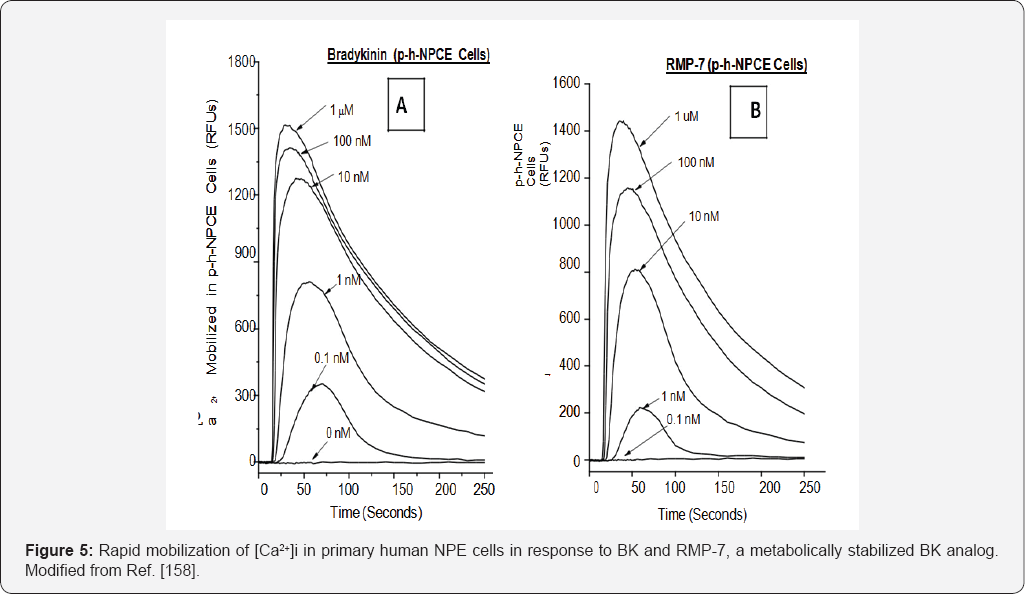
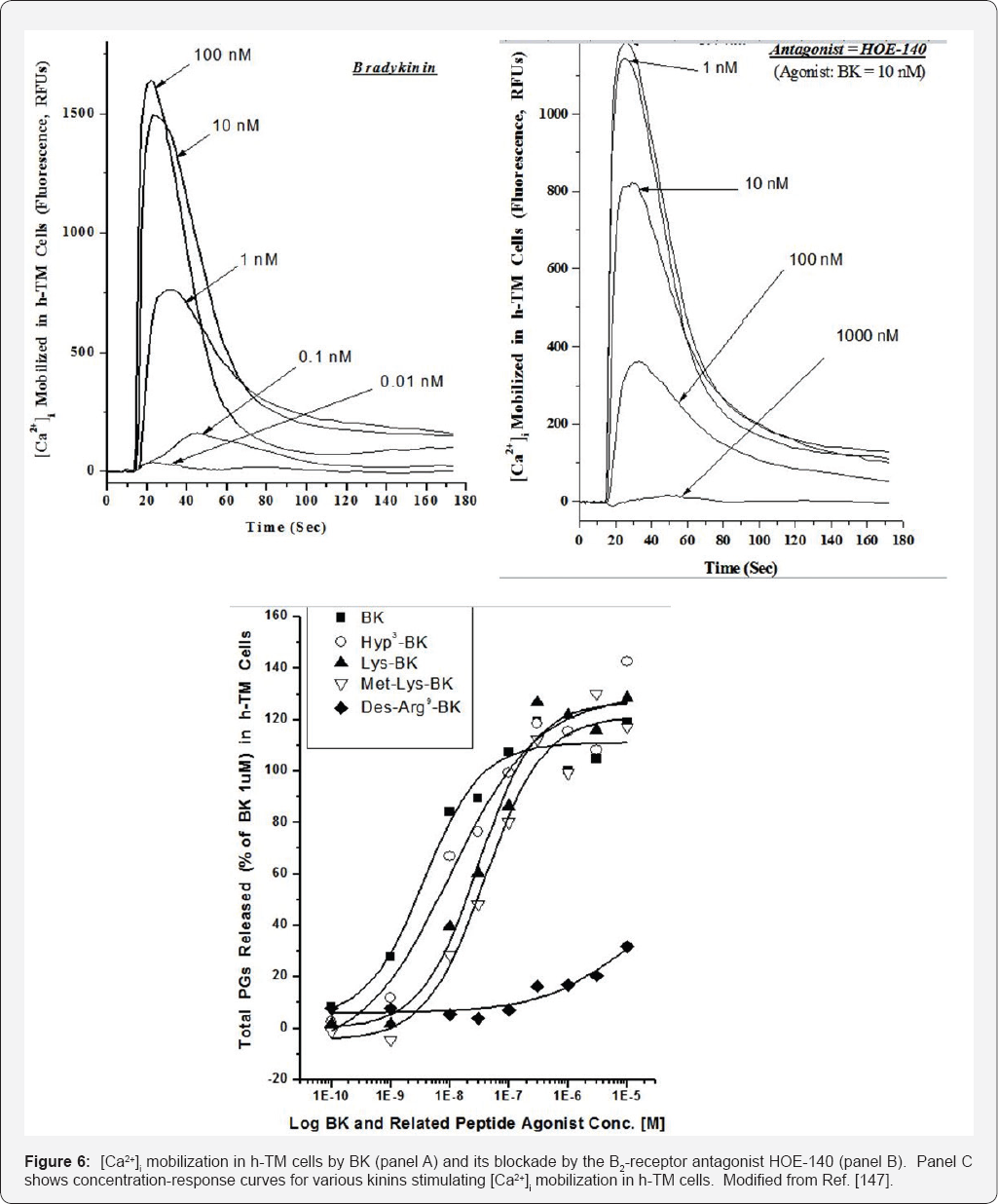
ocular cells mentioned above. To this end, primary h-NPE, h-CM and h-TM
cells were isolated and challenged them with BK, its many peptide
analogs and two non-peptide BK-mimetics, FR-190997 and BK2A78. Many of
these kininergic compounds stimulated the production of intracellular
inositol phosphates [
142,
156], and all peptide and non-peptide agents liberated endogenous intracellular Ca
2+ ((Ca
2+)i) from the endoplasmic reticulum in normal h-NPE, h-CM and h-TM cells [
146,
147,
158]
Figures 5,
7 (and in bovine TM cells [
155]) to varying degrees and with different relative potencies, a feature also observed in cells transfected with a cloned human B
2-receptor. Additionally, these BK agonists also activated extracellular-regulated kinases-1/2 in h-TM [
145] and h-CM [
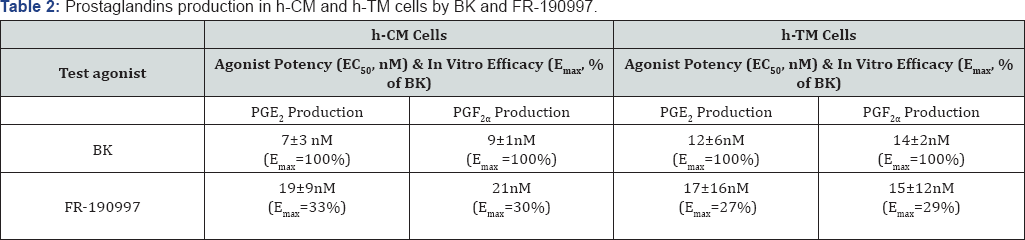
159] cells, and promoted the synthesis and secretion of PGE2 and PGF2a in the latter cells
Figure 7. Since Des-Arg
9-BK (a selective B
1-agonist) was always a very weak agonist in all these biochemical assays, and since two B
2-receptor
antagonists (H0E-140 and WIN-64338) potently blocked the responses
induced by BK, RMP-7 (a stabilized peptide analog of BK), FR-1909997 and
BK2A78 [
146,
147,
156,
158,
160,
161], the functionality and pharmacological identification of B
2-receptors
in these cells were completely confirmed. Additional studies in h-CM
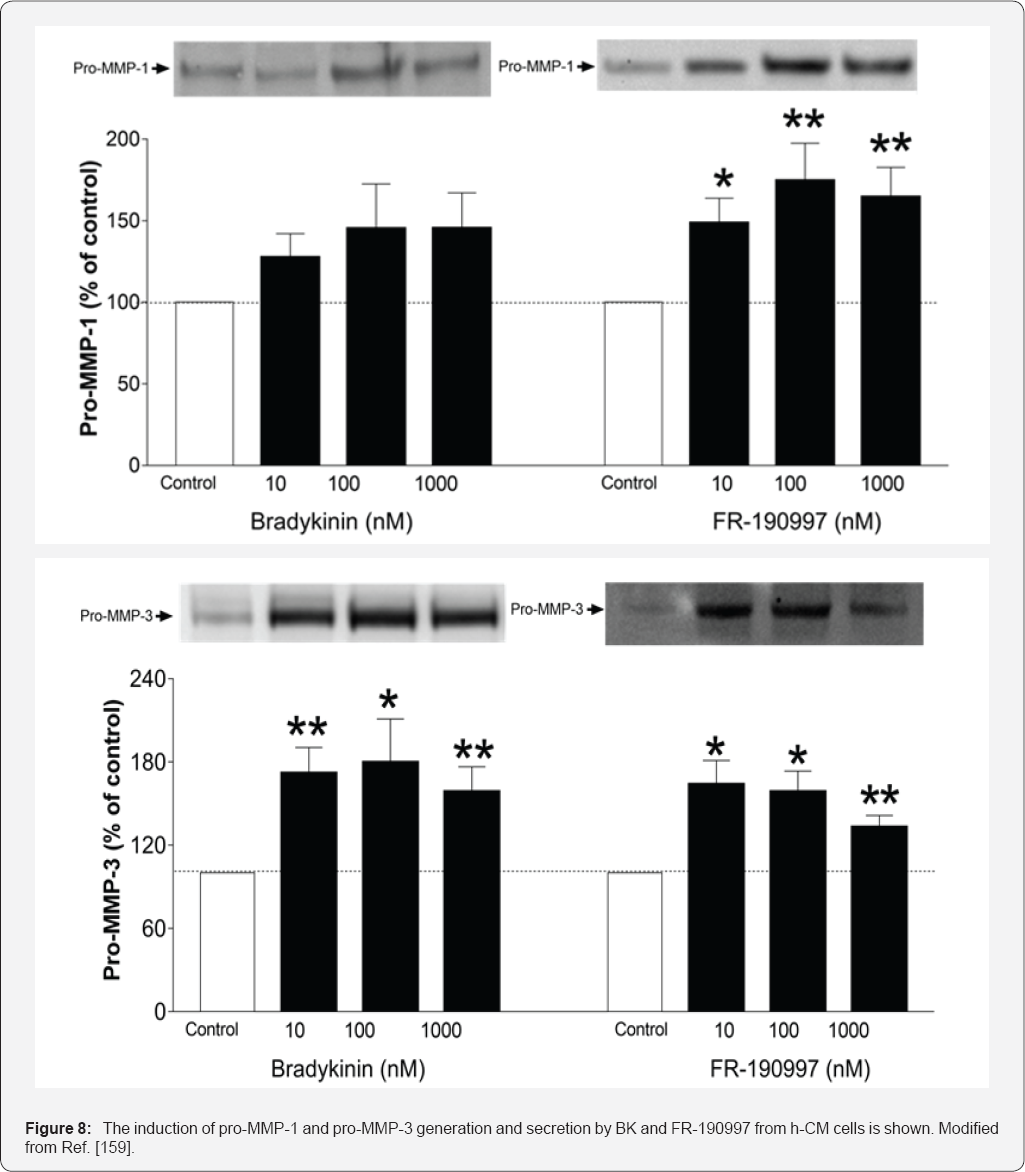
and h-TM cells indicated that BK and FR-190997 could also activate
intracellular signal transduction pathways to cause the release of
various matrix metalloproteinases [
106,
159]
Figure 8
that are known to digest extracellular matrix components such as
collagen and thus aid in the efflux of AQH from the anterior chamber to
lower IOP, a mechanism previously associated with ocular hypotensive
FP-class PGAs [1-3,8,9,53]. Interestingly, BK enhanced the production of
cAMP induced by PGE2 in h-TM cells [
162],
indicating that additional control of TM function by kinins was
possible through activation of adenylyl cyclase. The next exciting phase
of investigations tried to link these diverse biological actions of
kinins in isolated cells of an almost intact organ, in this case
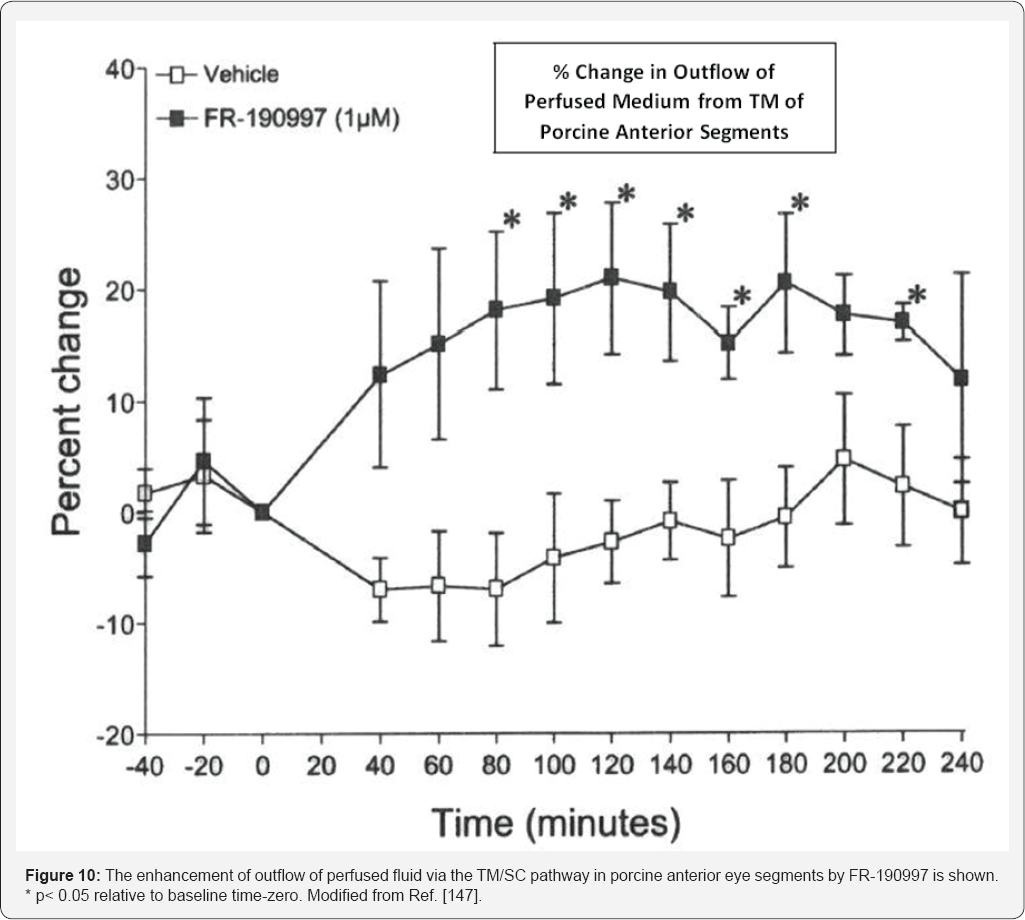
enucleated porcine and bovine anterior segments of the eye. While one
study initially showed a somewhat decrease in perfused fluid outflow in
response to BK in the bovine eye [
155], other studies by Webb et al. [
106]
showed that BK actually robustly stimulated outflow in bovine eyes as
did the BK mimetic FR-190997 in other independent experiments using
porcine eyes [
156]. Mechanistically Webb et al. [
106] also showed that this increased outflow by BK was mediated by B
2-receptor-induced secretion of MMP-9 since a B
2-antagonist and an MMP inhibitor abolished the effects of BK [
106].
The role of BK in modulating I0P was investigated in a
number of species.Topical ocular (t.o.) instillation of BK (50- 100|ig
in a 30|il drop) to Dutch-belted rabbits, mixed breed cats, mice, rats,
guinea pigs and cynomolgus monkeys (ocularly normotensive or
hypertensive) failed to consistently influence IOP to any significant
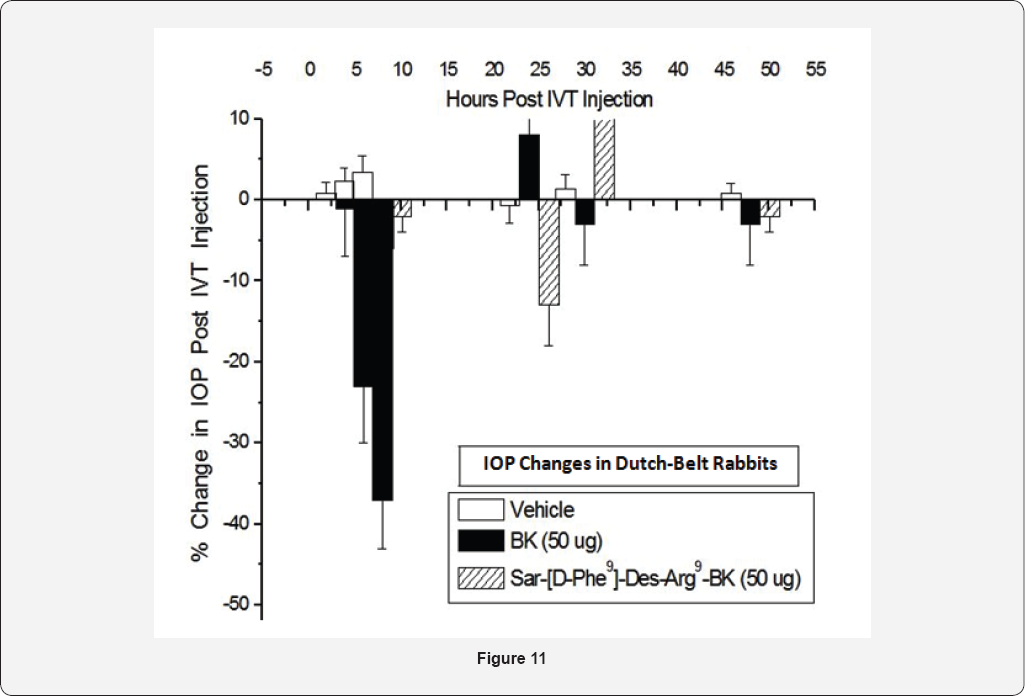
extent. However, intravitreal injection (ivt) of BK (50|ig) in eyes of
Dutch-Belted rabbits induced a robustdecrease in I0P up to 8 hrs
post-injection [
146]. Interestingly, both B
1-receptor-agonists, Des-Arg
9-BK and Sar(D-Phe9)-Des-Arg
9-BK injected ivt, did not alter I0P at all [
146,
147]. These data further substantiated the fact that only B
2- receptors are involved in lowering and controlling I0P without any contribution from B
1-receptors,
at least in the rabbit. Since ethically and economically, we could not
repeat it injection studies using BK in higher animals, and since
topical ocular BK was without effect, a different approach was
necessitated. Also, even though a metabolically stabilized peptide
mimetic of BK (RMP-7) is available, it is still too polar a molecule to
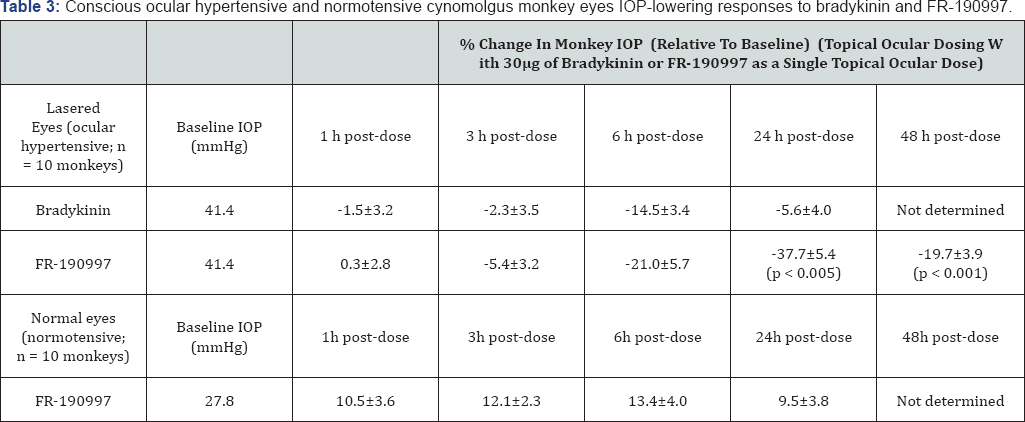
be used t.o. For such I0P modulation studies. However, a non-peptidic
hydrophobic BK-mimetic, FR-190997 formulated in a standard vehicle was
bioavailable when administered topically, and it potently and
efficaciously reduced IOP in the conscious ocular hypertensive monkey
eyes [
156,
160].
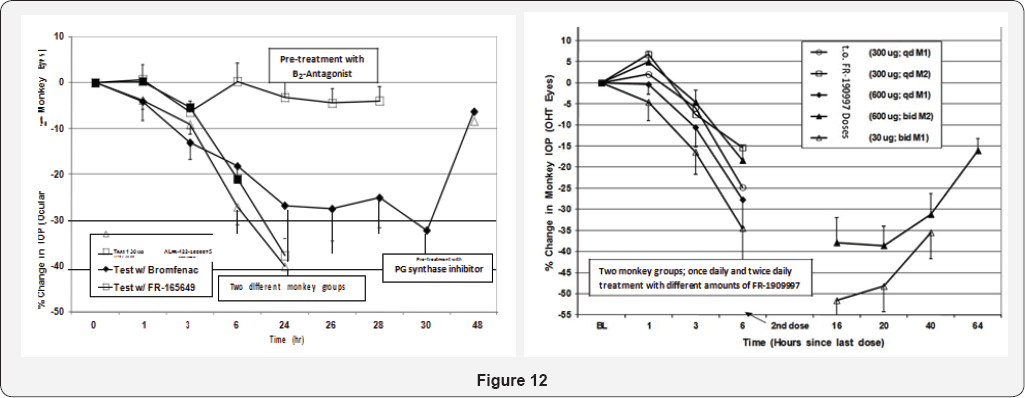
As little as 1|ig total t.o. Dose induced a 25% IOP reduction for up to
24-hrs after dosing, and dose-dependent reductions out to 48hrs
post-t.o. Dosing were possible with a 10|ig dose [
156,
160].
The ocular hypotensive effects of FR-190997 and BK2A78 were in the
realm of what the t.o. PG FDA-approved drugs like TRAVATAN® and Xalatan®
produce in the monkey-model and in humans, but only to 24hrs post-dose.
The fact that a non-peptide B
2- receptor antagonist
(FR-165649) completely prevented the I0P- lowering actions of FR-190997
in the 0HT monkey eyes strongly suggested that the B
2-receptor was mediating the I0P-lowering actions of FR-190997 [
156].
Furthermore, since FR-190997's ocular hypotensive effects were
significantly attenuated by prior treatment with a PG-synthesis
inhibitor (bromfenac) PGs were involved in mediating at least some of
the I0P-lowering activities of this BK-mimetic, this being akin to the
in vitro observations with FR-190997 [
156,
160]
and BK [145-147,162]. In ascribing possible mechanisms activated by
FR-190997 in its ability to reduce I0P in mildly-sedated 0HT monkeys, it
was discovered
that a predominant enhancement of uveoscleral outflow of AQH was
triggered by FR-190997 [
106,
156].
However, it would appear that in the porcine isolated anterior chamber
model, this compound (and BK in bovine) promoted fluid egress via the
TM/SC conventional outflow pathway [
106,
156].
It remains to be seen whether such observations of robust ocular
hypotensive activity of FR-190997 and BK2A78, along with other
non-peptide BK-mimetics, can be reproduced in OHT human patients. Since
FR-190997 [
160] and BK2A78 [
161]
caused minimal ocular discomfort in conscious Dutch-belt rabbits, mixed
breed cats, rats and monkeys after t.o. dosing, and produced no
observable systemic or local side-effects, such compounds represent
ideal new drug candidates worthy of pursuit in appropriate human
clinical trials for determining ocular hypotensive activity. Two
physiological observations noted that may limit the future utility of
such BK-mimetics to treat OHT/POAG are the apparent tachyphylactic
effects of FR-190997 in terms of IOP-lowering at relatively high doses,
and a mild anesthetic activity observed on cat corneal surface [
160].
However, whether these elements translate to the human ocular system
requires further study. As long as low pharmacologically-relevant t.o.
doses of FR-190997 and BK2A78 (and their analogs and derivatives [
162-
164])
are used t.o. in other animal models and in human subjects, it is
possible to avoid triggering the above-mentioned "adverse" effects.
Further studies on the ocular roles of BK and its analogs and mimetics
are eagerly awaited.
Clearly there are now several drugs approved for the
treatment of OHT/POAG and a number of new AQH drainage devices either
approved or on the horizon for the same purpose of lowering IOP It is
the issues of compliance, and the number and relative seriousness of the
side-effects, or ineffectiveness and complications of the procedures,
that continue to warrant hunt for newer more efficacious and more
tolerable medications. The latter quest has resulted in the recent
discovery of some new ocular hypotensive agents, including the first
generation Kenyan non-peptide mimetics such as FR-190997, BK2A78 and
their analogs [
162-
164].
The studies described in this review have clearly
shown the presence of various components of the kininergic system in
human and monkey ocular cells and tissues using a variety of techniques.
Furthermore, functionally active sub-types of BKreceptor (B
1- and B
2)
also are present in the ocular cells involved in AQH dynamics, Hence,
BK and its analogs and mimetics are able to generate a variety of second
messengers such as inositol phosphates and intracellular Ca
2+
in h-NPE, h-CM and h-TM cells. Activation of this signal transduction
pathway, then stimulates the production and secretion of PGs from these
cells. These PGs are pivotal in promoting the generation and release of
MMPs from CM and TM cells that digest extracellular matrix to create new
pathways for AQH to drain from the anterior chamber of the eye leading
to lowering of the IOP. Such duality of action of MMPs in response to BK
receptor activation probably explains the elevated TM/SC outflow and
increase of uveoslceral outflow observed after treatment with
FR-190997and the profound IOP-lowering that this compound produces [
156,
160]. These new non-peptidic kinin mimetic drugs [
156,
162-
164]
will hopefully inspire other researchers to use these as templates for
synthesizing next generation of ocular hypotensive agents, perhaps with
some secondary neuroprotective activity on top of the ocular hypotensive
properties. We all await the results of such new discoveries.
Author is an inventor or co-inventor of some granted
patents related to the use of BK agonists (peptide and non-peptide) for
treatment of glaucoma and the associated OHT, and these are cited in
this article. The author, is an adjunct professor at Texas Southern
University (Houston, TX) and at University of North Texas Health Science
Center (Fort Worth, TX), and has no other conflicts of interest to
declare. The intent of this review article is simply to share and expand
the knowledge of the ocular roles of Kinins and thus inspire further
research in this arena for the discovery of novel drugs and treatments
to help combat blinding diseases of the eye, especially OHT/POAG.